Description
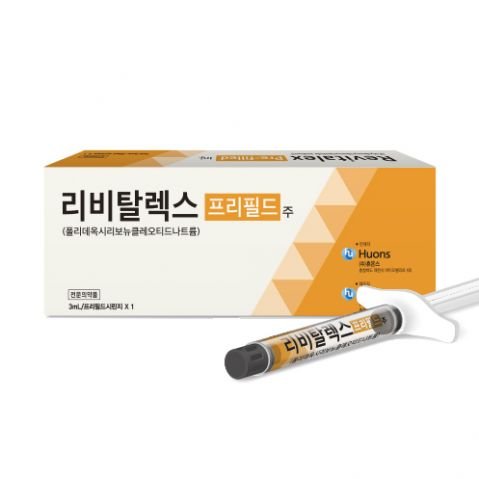
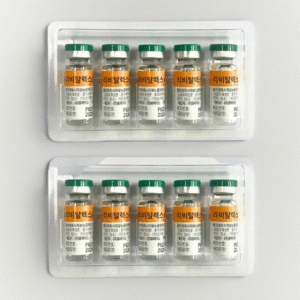
Product Name : Revitalex Prefilled Injection (Polydeoxyribonucleotide Sodium)
Product Category: Prescription Drug
Active Ingredient: Polydeoxyribonucleotide Sodium 5.625 mg / 1 syringe (3 mL)
Indications and Effects: Post-Skin Grafting Wound Management and Tissue Restoration
Appearance: A prefilled injection syringe consisting of a colorless, transparent plastic barrel filled with a clear solution and sealed with rubber stoppers on both ends.
Pack contains: 1 prefilled syringe (3 mL)
Storage Instructions: Store in a sealed container at room temperature.
Polydeoxyribonucleotide(PDRN) is administered as an injectable formulation, with each 3 mL syringe containing 5.625 mg of the active ingredient. It is primarily used to promote wound healing and tissue regeneration following skin graft procedures by stimulating fibroblast proliferation and facilitating tissue repair.
This compound exhibits the following therapeutic properties:
- Tissue regeneration: Enhances the production of growth factors and cytokines, accelerating the wound healing process.
- Anti-inflammatory effects: Suppresses inflammatory mediators, thereby reducing swelling and pain.
- Improved blood circulation: Promotes angiogenesis, ensuring adequate oxygen and nutrient delivery to the damaged area.
It is typically administered once daily via intramuscular or subcutaneous injection and should only be used under the supervision of a qualified healthcare professional.
Cautions to use
1. Contraindications
This drug should not be administered to patients with hypersensitivity to the active ingredient or any of the excipients contained in this product.
2. General Precautions
If hypersensitivity reactions occur following administration, discontinue use immediately and provide appropriate medical treatment.
3. Drug Interactions
No studies have been conducted regarding interactions between this drug and other medications.
4. Post-Marketing Surveillance in Korea
A post-marketing surveillance study was conducted over six years in Korea involving 609 patients for re-evaluation purposes. The incidence of adverse events, regardless of causality, was reported at 0.99% (6 out of 609 patients, 7 cases in total). Reported events included pruritic rash, oropharyngeal pain, vomiting, leukopenia, abdominal pain, diarrhea, and nausea—each occurring once. No serious or unexpected adverse events were reported.
※ Re-evaluation Safety Analysis Summary
An integrated analysis of domestic re-evaluation data and spontaneous adverse event reports for this drug, compared with adverse event data from all other approved pharmaceuticals in Korea at the time of re-evaluation, revealed no statistically significant increase in adverse event reporting for this product.
Stimulation of cell formation: PDRN promotes proliferation of human skin fibroblast. PDRN induces VEGF (Vascular Endothelial Cell Growth Factor), resulting in angiogenesis and improved blood flow. PDRN produces nucleotides and nucleosides, used when forming DNA and activating normal cell proliferation and growth. This regenerative product helps restore skin moisture levels, reduce pigmentation, shrink pores and increase collagen production, which depletes with age.
- Approved by the MFDS Korea (Ministry of Food and Drug Safety)
- Regenerative and stimulates fibroblast formation in the skin
- Quality manufacturing process through high-temperature extraction and purification
- Loaded with good fats and co-factors, PDRN speeds healing and reduces inflammation after skin procedures.
- Includes vascular endothelial growth factor (VEGF), which stimulates new blood vessels and improves blood circulation
- Stimulates the synthesis of nucleotides and nucleosides used in DNA formation, activating the normal formation and growth of cells
- High PDRN concentration: 50-1500 kDa (DNA) and 5,626mg polydeoxyribonucleotide 5.6%
Made in South Korea

 Estimated Delivery: Domestic shipping usually takes about 1 to 3 days.
Estimated Delivery: Domestic shipping usually takes about 1 to 3 days.






There are no reviews yet.