Description
DemiOS is a bone graft material formulated by combining demineralized bone matrix (DBM) with biocompatible, water-soluble polymer Poloxamer gel. This combination enhances both osteoconductive and osteoinductive capabilities, meaning it promotes mesenchymal cell proliferation and differentiation into osteoblasts, ultimately stimulating normal bone formation.
Composition and Characteristics of the Product
DemiOS is an adhesive bone filler. The main components of the filler include demineralized bone matrix (DBM) powder particles derived from human tissue, combined with Poloxamer hydrogel carrier in a paste formulation. It is applied by injecting the required amount into the bone defect site.
The DBM particles mixed with Poloxamer hydrogel carrier fill voids and gaps in the bone, promoting new bone formation.
Performance and Intended Use
DemiOS is a bone filler designed for skeletal tissue restoration, particularly in areas where bone structural stability is lacking, such as distal ends, spine, and pelvis bone defects. It serves the following purposes:
– Complementing and reconstructing autograft bone
– Filling inside interbody fusion cages for spinal fusion
– Supporting fusion and filling bone defects of various shapes and sizes
Pre-use Preparation Guidelines:
– Check for any abnormalities in the packaging condition and verify the presence of the sterility indicator to confirm sterility.
– Verify the expiration date before use.
– Consider potential allergic reactions or other responses to the product’s material.
– The practitioner must be fully familiar with handling and operation before using the product.
– Read the instructions carefully before use.
Usage Instructions and Operation Sequence
To minimize the risk of postoperative complications, sterile techniques must be maintained. The required amount depends on the size of the bone defect, and rehydration is not necessary before use.
1. Remove the outer double or triple packaging and hand over the inner packaging to a surgically sterile team member, ensuring it remains uncontaminated.
2. Check for any damage to the inner packaging.
3. Remove the inner package and take out the syringe.
4. Detach the protective cap from the syringe tip.
5. Slowly push the plunger to fill the intended bone graft site with the contents.
6. Use sterile instruments to pack the material into the bone defect.
7. Do not reuse any remaining contents after a single application.
Storage and Maintenance After Use
This product is sterile, so it must be used immediately after opening. Any unused portion should not be stored for later use and must not be reused.
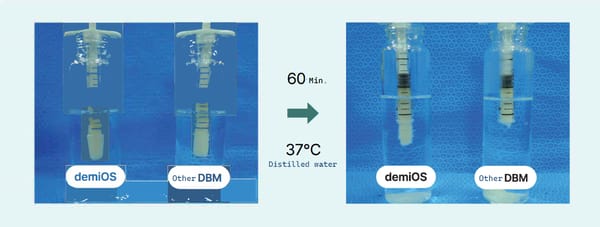
In a body-temperature environment (36°C), viscosity increases, so when injected into bone defect sites, DBM does not disperse or get washed away by bodily fluids, preventing it from dislodging from the implantation site.
Precautions During Use
Clinical trials have not shown any fatal adverse effects from this product. However, in addition to common oral procedure-related reactions, the following potential adverse reactions may occur:
– This product must only be used for a single patient.
– It should not be used for non-medical purposes and should only be handled by qualified medical professionals.
– Use only products without defects or damage in packaging or appearance.
– If the packaging is found damaged upon receipt, do not proceed with implantation and immediately return it to the manufacturer.
– Read the instructions carefully before use.
– The implanting physician must discard or return products that are unsuitable for implantation, unused after opening, or found damaged.
– Ensure the patient has no known allergies to the reagents or solutions used in the processing of the product.
– Do not implant if the site of implantation is infected.
– Do not re-sterilize and reuse the sterile product.
– Although the antigenic response has been removed during manufacturing, occasional reactions may still occur.
– Since this product contains human tissue, physicians must always consider the potential risk of infectious disease transmission.
– Store at room temperature (15–30°C) as refrigeration or freezing may cause phase separation.
※ This product is a “medical device”. Please read the “precautions for use” and “instructions for use” carefully before handling.
What B-TRP (Bone Tissue Regeneration Peptide) Means in DemiOs?
- The product is a bone graft filler, and the main emphasis is on osteogenesis—the formation of new bone.
- B-TRP in DemiOs is described as a bioactive peptide that promotes mesenchymal cell differentiation into osteoblasts (bone-forming cells).
- It works synergistically with demineralized bone matrix and Poloxamer gel to support bone regeneration.


 Estimated Delivery: Domestic shipping usually takes about 1 to 3 days.
Estimated Delivery: Domestic shipping usually takes about 1 to 3 days.




There are no reviews yet.